


Pounds in biological samples is the serial dilution assay, in which measurements are taken at several different dilutions of a sample, giving several opportunities for an accurate mea-surement.Currently, serial dilution is a standard tool in the fields of toxicology and immunology.Our experience is in.Ī tenfold dilution for each step is called a logarithmic dilution or log-dilution and a 3.16-fold (100.5-fold) dilution is called a half-logarithmic dilution or half-log dilution. From this, a calculation of viable cells in the original suspension can be made, as a colony picked for pure culture. In microbiological technique, serial dilutions are used to obtain a culture plate that yields a countable number of separate colonies. Serial dilution a set of dilutions in a mathematical sequence. Serial Dilution Besides the more conventional uses described above, serial dilution may also be used to reduce the concentration of microscopic organisms or cells in a sample. Serial dilutions are used to accurately create highly diluted solutions as well as solutions for experiments resulting in concentration curves with a logarithmic scale. Usually the dilution factor at each step is constant, resulting in a geometric progression of the concentration in a logarithmic fashion. There are two situations where serial dilutions should be used rather than parallel dilutions: FIRST : Use a serial dilution when you need several solutions of the same solute and there is a constant dilution factor. Difficulty Procedure: Medium Concept: Easy Concept Serial dilution is the stepwise dilution of a substance in solution. Application Experimental sciences include biochemistry, pharmacology, and physics as well as in homeopathy.
Serial Dilution Definition A method used to stepwise dilute substance into solution with constant dilution factor in each step. And u inoculate 0.1 ml in your medium.so supposed the final volume of your medium is 1ml right? Regarding the example 2 given, you dilute a sample (10 ml the final volume) 10x10x10=10 to the power of 3. It is true that they have learned it in high school, but most of the time they will forget. Indeed, in this case, it is not necessary to take into account flow rate discrepancies as in the calibration approach.Property of Yulia- i'm very thankful to you to provide the easier 's true that many undergrad students have problem in dilution.and the worst thing is if the lecturers/tutors assume that the students are familiar with the concept. We also demonstrated that compared to the calibration curve approach, the standard addition mode is less complex to operate. When using the on-chip calibration and on-chip standard addition method, we reached concentration estimation better than 5%. Moreover, the microfluidic approach is compatible with the on-chip calibration of sensors simultaneously to the analysis, therefore preventing problems due to sensor response deviation with time. Operating a quantification in this microfluidic parallel approach rather than in batch mode allows a reduced analysis time to be achieved. A ten-foId serial dilution couId be 1 M, 0.1 M, 0.01 M, 0.001 M. Usually the dilution factor at each step is constant, resulting in a geometric progression of the concentration in a logaritmic fashion.
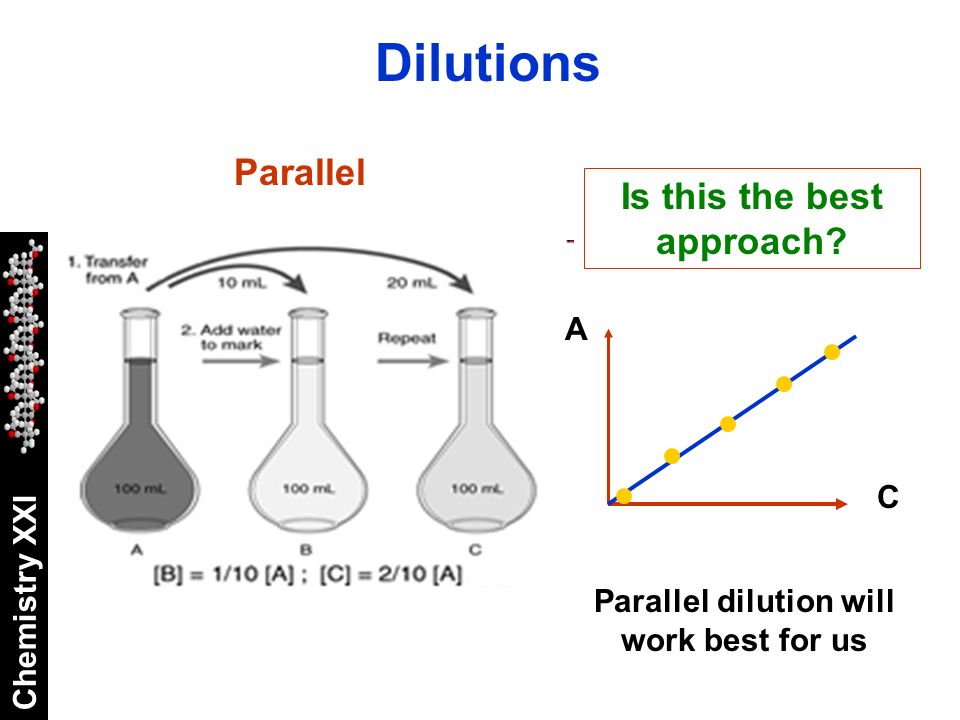
#Serial Vs Parallel Dilution series#
So serial dilutions are done in a particular series and everything can be in a series. This device was first characterized by fluorescence microscopy and then evaluated with a model electroactive molecule such as Fe(CN 6) 4−. Serial Vs Parallel Dilution Method Series And Everything. This device integrated a network of Au electrodes within a microfluidic structure designed for automatic preparation of a series of solutions containing an electroactive molecule at a concentration linearly decreasing.
This paper describes a microfluidic device fabricated in poly(dimethylsiloxane) that was employed to perform amperometric quantifications using on-chip calibration curves and on-chip standard addition methods.


 0 kommentar(er)
0 kommentar(er)
